Detection of New SARS-COVID19 Variants on PCR Testing
Confirmation of detectability of the new mutated variants of SARS-CoV-2 using Seegene AllplexTM 2019-nCoV PCR Assay at Pathologists Lancet Kenya_PLK
We are pleased to confirm that the new mutated variants of SARS-COV-2, including the B.1.1.7 UK variant and the SA variant, can be detected with the AllplexTM 2019-nCoV PCR Assay that is used at Pathologists Lancet Kenya_PLK.
*Specialized in-silico analysis by Seegene has confirmed that the COVID-19 PCR test covers the new variants of SARS-CoV-2 virus specifically including the B.1.1.7 lineage that emerged in the United Kingdom (UK variant) and also the B.1.351 lineage that emerged in South Africa (SA variant).
The Seegene AllPlexTM Assay is able to detect these variants because the assays are designed to detect individual multiple gene targets of SARS-CoV-2 simultaneously.
It therefore means that we are rest assured of being able to diagnose those infected with COVID-19 using PCR test on swab samples collected and tested with the highly sensitive and specific PCR assay employed at Pathologists Lancet Kenya_PLK - as the assay is able to detect both the old and the SARS-CoV-2
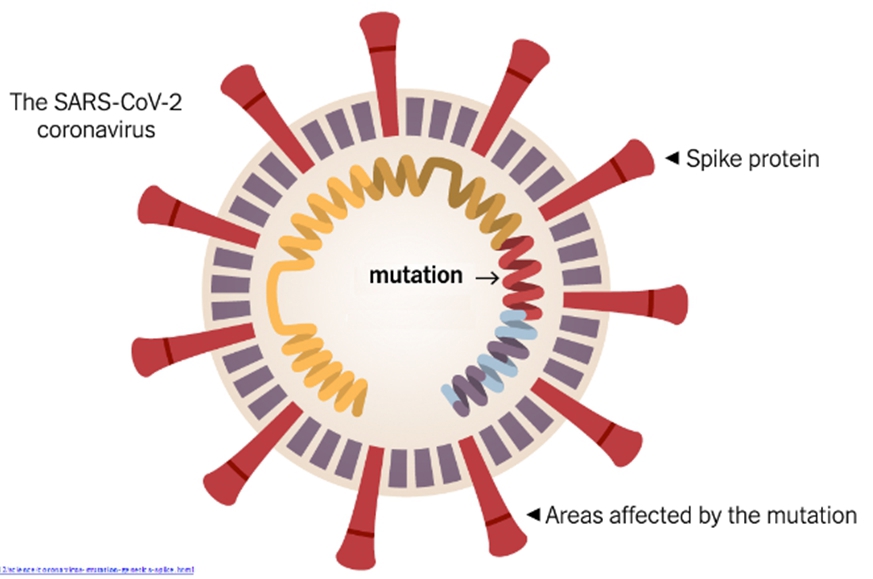
*The in-silico analysis showed that no mismatches were found in RdRP gene region, while few mismatches were found in the S gene region, but despite the few mismatches, the overall detectability of Seegene COVID-19 assays may not be affected by these mutations. Whereas the sensitivity variances can be affected by the mismatches, these are expected to be limited because of the complimentary multiple oligonucleotides(oligo) for each gene region in the assays. Further detailed information about the in-silico analysis can be availed from Seegene.
ABOUT THE SARS-COV-2 RT-PCR TEST AT PATHOLOGISTS LANCET KENYA
At Pathologists Lancet Kenya_PLK we've exclusively used the Seegene AllplexTM 2019-nCoV assay since April 2020 and conducted over 100,000 tests as at 25th of January 2021.
The Seegene AllplexTM assay is a reverse-transcriptase (rt) real-time (RT) polymerase chain reaction (PCR) that is a nucleic acid amplication test (NAAT) for detection of viral RNA for SARS-CoV-2 the virus that causes COVID-19.
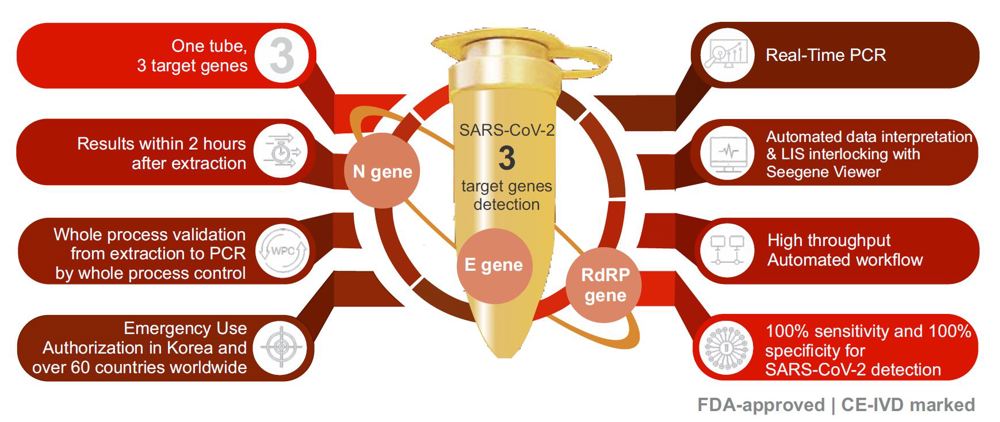
The Seegene AllplexTM PCR assay has a unique feature that identies 3 different target genes (E, RdRP and N genes) in a single reaction tube which allows for highly accurate results and maximizes the throughput for high volume testing.
The Seegene AllplexTM RT-PCR assay is FDA-approved and has been independently evaluated by FIND “Foundation for Innovative New Diagnostics” scoring it at 100% sensitivity and 100% specificity
Technical information on the SARS-COV-2 RT-PCR Test at Pathologists Lancet Kenya_PLK
- The Seegene Allplex rtRT-PCR assay is an automated molecular test at PLK that utilizes three target genes protocol (rather than two or single gene assay protocol) in deciding the SARS-COV-2 status on a sample analyzed in a single reaction tube as multiplex PCR test per sample, thus serving as both screening and confirmatory test.
- The interpretation of results is as per manufacturer's recommendation and in-line with WHO guideline that “In areas where COVID-19 virus is widely spread a simpler algorithm might be adopted in which, for example, screening by rtRTPCR of a single discriminatory target is considered sufcient (The viral genes targeted so far include the N, E, S and RdRP genes)”.
- The Segeene Allplex assay is duly approved by Ministry of Health's regulatory bodies and validated against NIC laboratory and Lancet Laboratories South Africa
- The PCR tests at PLK's Main Lab, which is registered and licensed as a National Class F Reference Laboratory by KMLTTB, are conducted by trained medical laboratory technologists who are also licensed by KMLTTB, with supervision from a PhD Molecular Scientist Doctor and several pathologists who work as a team to check and ensure accuracy of results.
- Automated data interpretation and LIS interlocking with the proprietary Seegene Viewer software enables a seamless and rapid review of results with intelligent interpretation.
- Accurate interpretation of the SARS-COV-2 PCR test results is enabled by the Seegene Viewer software which is designed to automate data analysis for multiplex real-time PCR assay allowing identication and differentiation for both Ct value of multiple targets in a single channel as well as melting curve analysis.
- The results from automated interpretation on Seegene Viewer are further reviewed by a laboratory technologist and pathologist on the Seegene Viewer before results are interfaced to the LIMS and additionally reviewed for release of the report with applicable interpretative comments as relevant based on clinical and epidemiological information.
- At PLK we interpret and report any viral target gene detected with a Ct lower or equal to the cut-off of 40 as positive. Ct > 40 is considered negative.
- Single gene results with high Ct values (low viral RNA levels) are issued with comments to highlight the possible implication of the results which include a sample at concentrations near or below the limit of detection of the target gene not amplied or a mutation in the corresponding target gene not amplied.
- From experience, the cases with single-gene only or low viral load are often seen in late-stage of infection but can also be seen early in the infection, thus it is important to correlate clinically and epidemiologically
- Our PCR laboratory has been continuously undertaking internal quality control and also participated in local and international external quality assurance (EQA) since May 2020
- We have consistently scored 100% in all the different EQA programs in over 10 consecutive cycles that include the LGC Thistle, NPHLS-MOH, KEMRI and RCPAQA prociency testing programs
- In addition, PLK has undergone onsite inspection and audit by experts from Kenya Medical Practitioners and Dentists Council (KMPDC) and Kenya Medical Laboratory Technicians and Technologists Board (KLMTTB) whose reports confirmed the accuracy and validity of our test results
- Our laboratory was the rst in Africa - in August 2020, and so far the only in the region, to receive ISO15189 accreditation specic for COVID-19 PCR test.
References
- https://www.fda.gov/media/137178/download
- https://apps.who.int/iris/handle/10665/330676
- http://www.seegene.com/covid19_detection
- http://www.seegene.com/assays/allplex_2019_ncov_assay
